Valvuloplasty Pack TAVI
BRIEF DESCRIPTION
Cardiac catheterisation, valvuloplasty
PRODUCT CODE
05-113937ΑΒ-03
COUNTRY OF ORIGIN
The Netherlands
TRADE NAME
CARDIO PACK 4 TAVI
APPROVAL BY THE NATIONAL ORGANIZATION FOR MEDICINES (NOM)
2820007946167
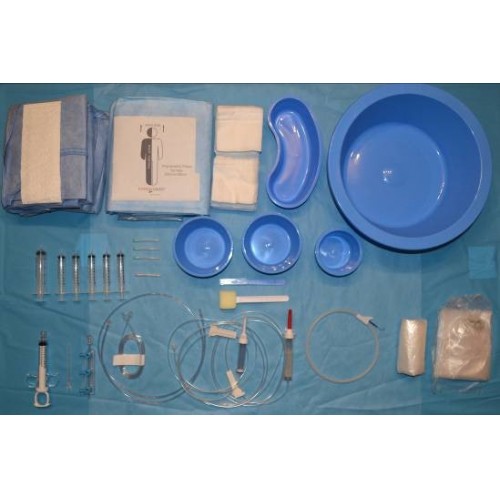
CARDIO PACK 4 TAVI is designed to cover the cardiac catheterisation lab during valvuloplasty. It is a complete pack, made of high-quality materials that offers everything needed to prepare the operating table. The manufacturer Medica Europe B.V. states that all individual items are collected from the USA, the UK and European countries and packaged in the Netherlands following strict quality control based on the MDR standard.
The pack includes the following materials:
|
S/N |
DESCRIPTION |
UNIT |
|---|---|---|
|
1 |
Transparent protective cover 102Χ51cm, round |
1 |
|
2 |
Transparent protective cover 91Χ102cm, square |
1 |
|
3 |
Round Bowl 250ml, Blue |
1 |
|
4 |
Round Bowl 500ml, Blue |
2 |
|
5 |
Kidney Dish 700ml, Blue |
1 |
|
6 |
Special sponge with plastic handle 15cm, for Betadine of foerster clamp type |
1 |
|
7 |
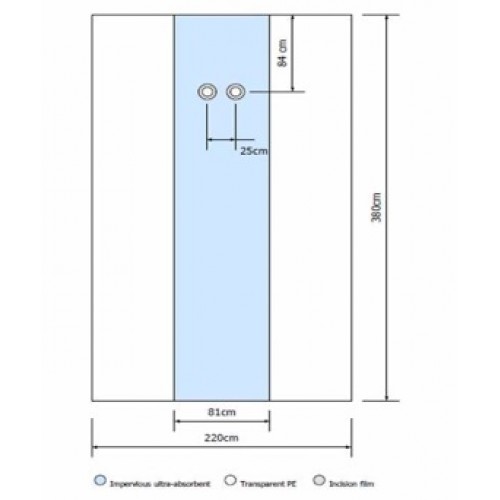
DRAPE FEM 3PLY radiographic table drape with 2 openings for femoral access and transparent along both sides 380x220cm, Blue |
1 |
|
7.1 |
DRAPE FEM/RADIAL 3PLY radiology table drape with 4 openings for femoral and radial access and transparent along both sides 330X220cm, Blue |
1 |
|
8 |
Drape with opening 2ply 75Χ90cm, with fenestration 10cm, Blue |
1 |
|
9 |
10x10cm Gauze, 12ply |
50 |
|
10 |
Medical gown size: XL lined and hypoallergenic |
3 |
|
11 |
Hand towel 42.5X30.5cm |
3 |
|
12 |
PVC pressure connector 148cm, male/female |
2 |
|
13 |
3-way right (multi-tap) manifold, with handles, off, (OB, 500 psi) |
2 |
|
14 |
Infusion device with drip chamber |
2 |
|
15 |
Scalpel with handle No.11 |
2 |
|
16 |
3way 1200 psi OFF, RA |
1 |
|
17 |
Silk Suture 2/0 2.75cm |
1 |
|
18 |
20ml Syringe, Luer slip |
7 |
|
19 |
10ml coronary angiography syringe (thumb ring, finger rings) with stopper and rotating luer lock adapter (Socorex type) |
2 |
|
20 |
Surgical Table Cover 195Χ150cm, (waterproof, lined, absorbent), Blue |
1 |
|
20.1 |
Tray 28Χ25Χ5cm, Blue |
1 |
CARDIO PACK 4 TAVI is available sterile and in disposable packaging, indicating Lot number and Reference for easier tracking of the material. It has ISO 13485:2016, EN ISO 13485:2016 & CE-MDR approval.
 English
English Greek
Greek